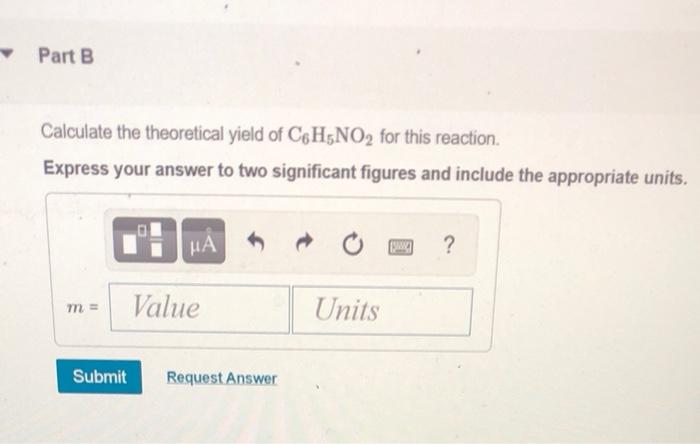
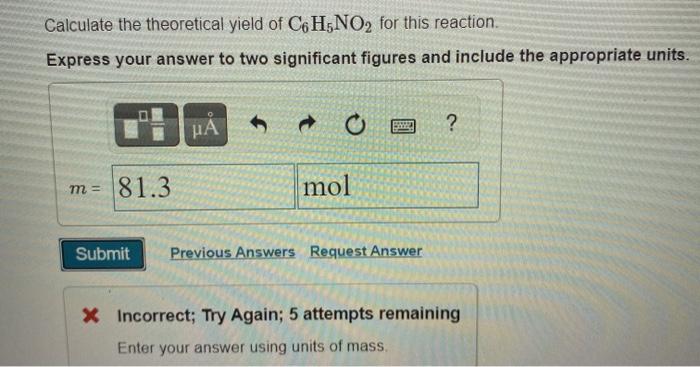
Calculate the Theoretical Yield of C6h5no2 for This Reaction
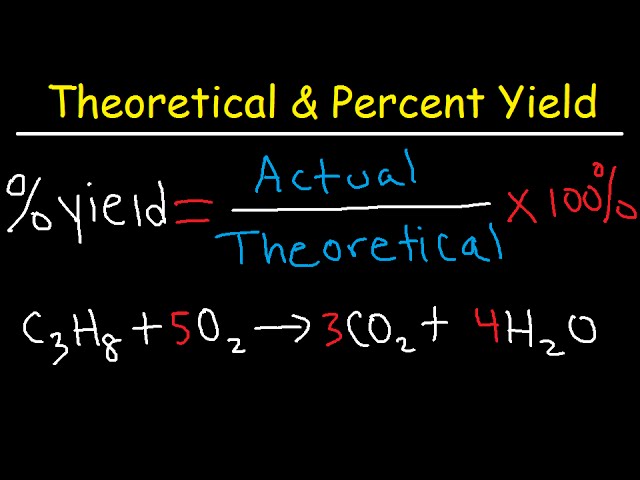
This chemistry video tutorial explains how to calculate the percent yield actual yield and theoretical yield of a product produced in a chemical reaction gi. From the experiment we already know the ACTUAL YIELD 316 g.
How To Calculate Theoretical Yield And Percent Yield Youtube
The amount of sugar in.
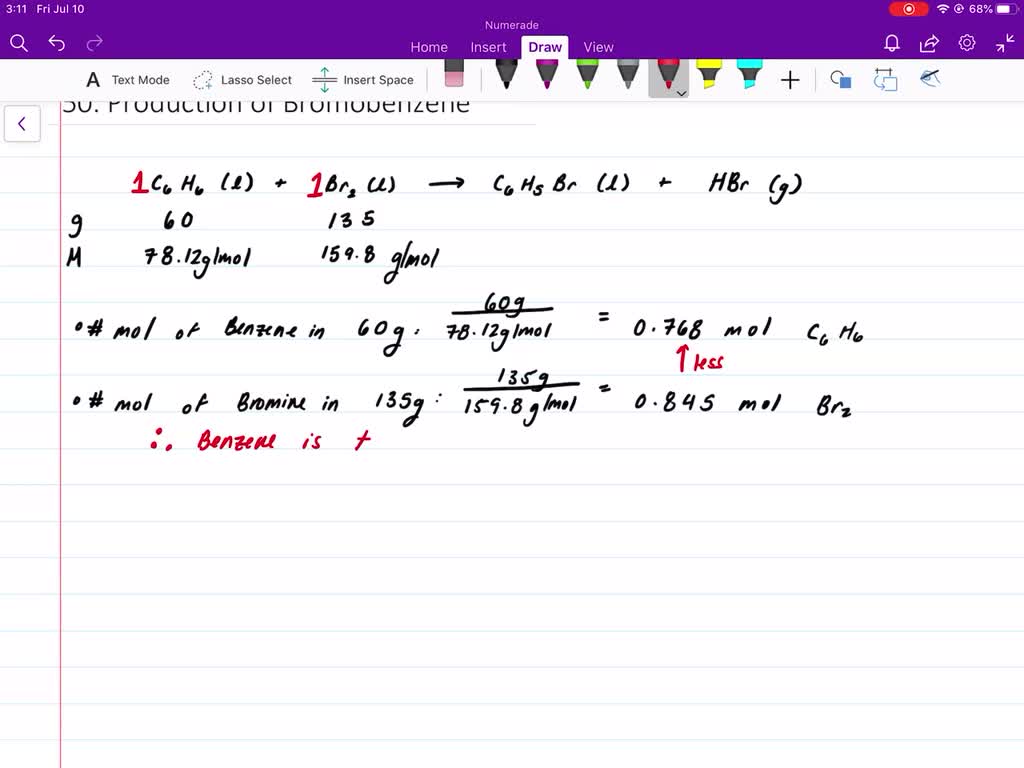
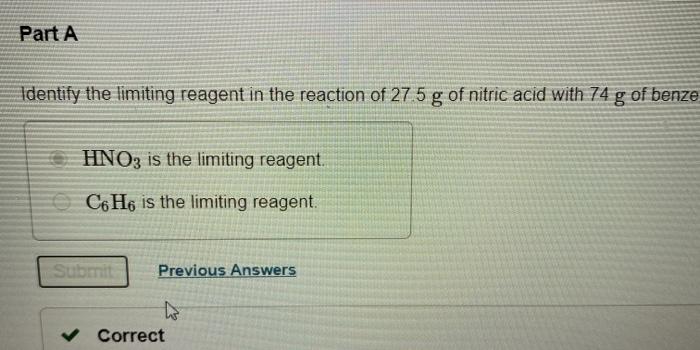
. Since the value of actual yield is usually less than the theoretical yield. HNO3 is the limiting reagent. Nitrobenzene C6H5NO2 is prepared from benzene C6H6 and nitric acid HNO3.
Once you convert 280 g HNOs and 74 g Cofle into the number of moles of each using their molar mass determine which produces less product Since the coeficientsn the balanced chemical equation are all 1 in this specific case you carn directly compare the number of. How Do You Calculate Theoretical Yield. Solute is the substance present in the smaller amount.
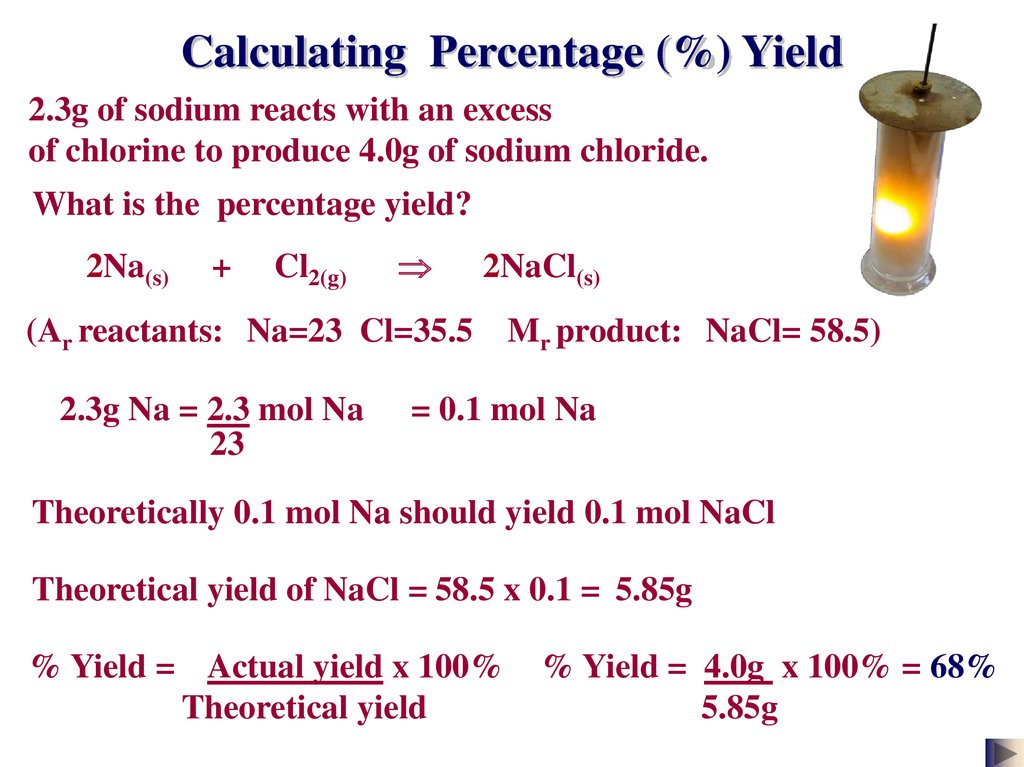
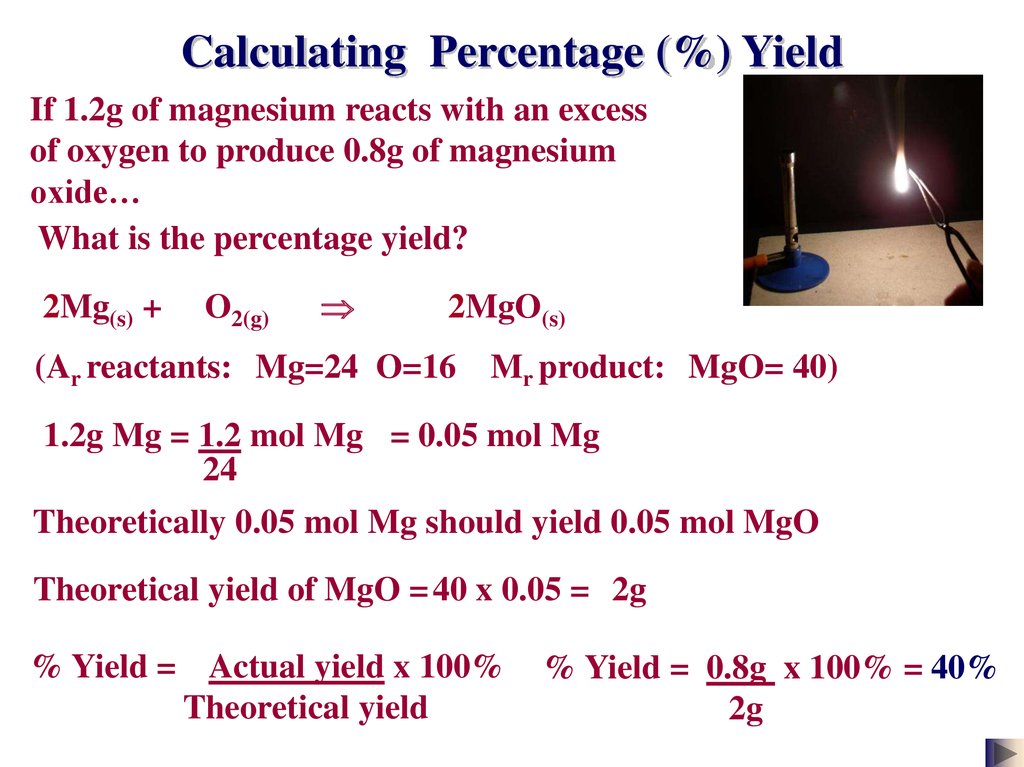
First we will calculate the theoretical yield based on the stoichiometry. Mass of ceKClO_3 400. Percentage yield of NaCl 850 grams 993 grams 100.
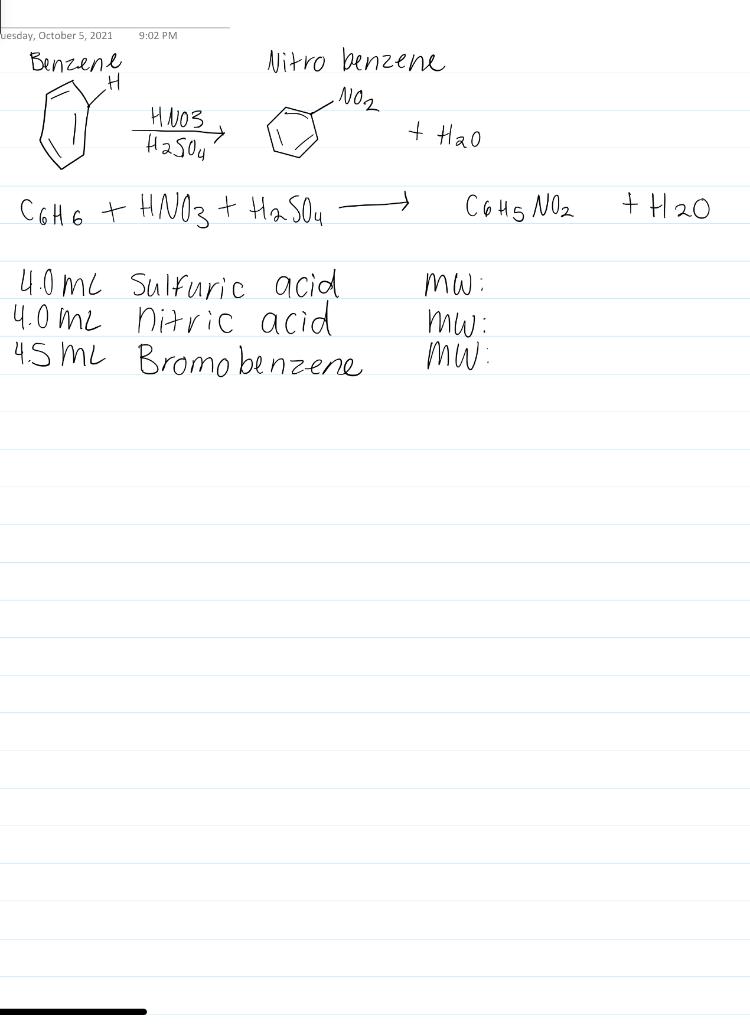
The other product of the reaction is water. C6H6 lHNO3 aqC6H5NO2 lH2O. Actual yield is said to be the mass of ammonia that is actually produce during the chemical reaction.
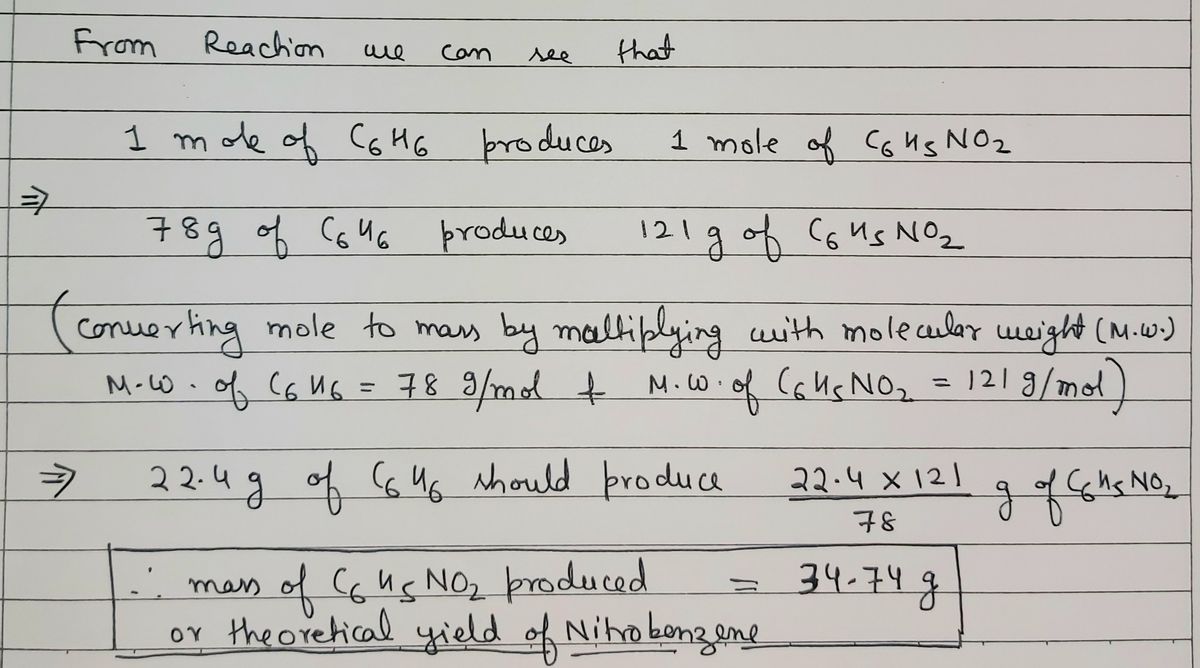
C6H5NO2 l H 2 Ol The limiting reagent is the reactant that limits the amount of product produced. 1 mole of C6H6 yields 1 mole of C6H5NO2. 1099 g N a B r.
View 13docx from CHEM 203 at Community College of Baltimore County. Use the molar mass of the product to convert moles product to grams of product. Write down the balanced chemical equation.
The percentage yield is the actual yield 316 g in this case divided by the theoretical yield calculated above times 100. Calculate the maximum theoretical yield of calcium oxide that can be produced from 250 g of calcium carbonate. We must CALCULATE the theoretical yield.
We see there is expected to be 1452 grams of sodium bromide product. Calculation of theoretical yield. Percentage yield of NaCl 8559.
So to stop you from wondering how to find theoretical yield here is the theoretical yield formula. The reaction involves two reactants C6H6 and HNO3 that react in a 11 ratio. Collected from the reaction what is the percent yield.
Yield actual yield theoretical yield 100. Grams product grams reactant x 1 mol reactantmolar mass of reactant x mole ratio productreactant x molar mass of product1 mol product For a theoretical yield example assume we have 20 grams of hydrogen gas and hydrogen gas has a molar weight of 2. Molar mass of H 2 gas 2 grams.
The reaction of 250 g benzene C6H6 with excess HNO3 resulted in 214 g C6H5NO2. From the reaction equation. Mm 53 gg.
Calculate the theoretical yield for this reaction. Hence 019 moles of C6H6 also yields 019 moles of C6H5NO2. Cas 2 H2Ol CaOH2aq H2g 840 123.
Textg Mass of O 2 collected 149g. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Up to 256 cash back Nitrobenzene C6H6NO2 is used in small quantities as aflavoring agent or in perfumes but can be toxic in large amountsIt is produced by reaction of benzene C6H6 with nitric acidC6H6lHNO3aqC6H5NO2lH2O.
To calculate percent yield you simply take actual yield 1099 grams of sodium bromide divided by the theoretical yield 1452 grams of sodium bromide. Grams product grams reactant x 1 mol reactantmolar mass of reactant x mole ratio productreactant x molar mass of product1 mol product The theoretical yield of our reaction is calculated using. The concentration of a solution defines the amount of solute dissolved in the solvent.
Terms in this set 92 C6H6 lHNO3 aq C6H5NO2 lH2O l Identify the limiting reagent in the reaction of 272 gg of nitric acid with 77 gg of benzene. Then the percent yield would be. C6H6 lHNO3 aq C6H5NO2 lH2O l Calculate the theoretical yield of C6H5NO2C6H5NO2 for this reaction.
CaCO 3 rightarrow CaO CO 2. C6H6 HNO3. Theoretical yield of ammonia NH3 is said to be the mass of product predicted by the balanced chemical equation for the reaction.
Nitrobenzene C6H6NO2 is used in small quantities as a flavoring agent or in perfumes but can be toxic in large amounts. What is the percent yield for the reaction. The molar mass of C6H5NO2 Nitrobenzene is.
3999 g m o l 1452 g N a B r. You are given the masses of both the reactants 277 g HNO3 and 77 g C6H6. Solvent is the substance present in the larger amount.
Number of moles in 15 g of benzene 15 g 78 gmol 019 moles. In aqueous solutions the solvent is water. It is produced by reaction of benzene C6H6 with nitric acid.
This is the reactant that produces fewer moles of the product when it completely reacts. Lets assume that you obtained an actual yield of 850 grams. The basic equation is.
What is the percentage yield. Use the moles of nitrobenzene and the molar mass of nitrobenzene to find the mass of benzene that should have been produced the theoretical yield. The formula for calculating the percent yield is.
Actual yield of ammonia NH3 408 g given in the above question 2. Mass of product molecular weight of product moles of limiting reagent in reaction stoichiometry of product where. Percentage yield mass of actual yield mass of theoretical yield 100.
Identify the given information and what the problem is asking you to find. Number of moles of 18 g of C6H5NO2 18g123 gmol 015 moles. In chemistry theoretical yield is a term that describes the amount of product that would result from a chemical reaction assuming that chemical reaction completesA chemical reaction is only considered complete when the entire limiting reactant is used up and it is impossible to create more products from the remaining chemicals.
Up to 256 cash back calculate the theoretical yield of C6H5NO2 for this reaction. 224 g 316 g ACTUAL. Calculate the theoretical yield for this reaction.
Using the following balanced equation calculate the percent yield for the reaction. Molar Mass of Nitrobenzene C6H5NO2. Moles of limiting reagent in reaction mass of limiting reagent molecular weight of limiting reagent stoichiometry of limiting reagent.
9 Concentration of Solutions Solution is a mixture of two or more substances dissolved in another.
The Ideal Gas Equation Prezentaciya Onlajn
Solved Nitrobenzene C6h5no2 Is Used In Small Quantities As Chegg Com
Calculate The Limiting Reactant And Theoretical Yield Chegg Com
The Ideal Gas Equation Prezentaciya Onlajn
Solved The Reaction It Is Asking About In The Picture Is Chegg Com

How To Calculate Theoretical Yield 12 Steps With Pictures

Solved Complete These Theoretical Yield Calculations 5 2 Chegg Com
Calculate The Theoretical Yield Of C6h5no2 For This Chegg Com
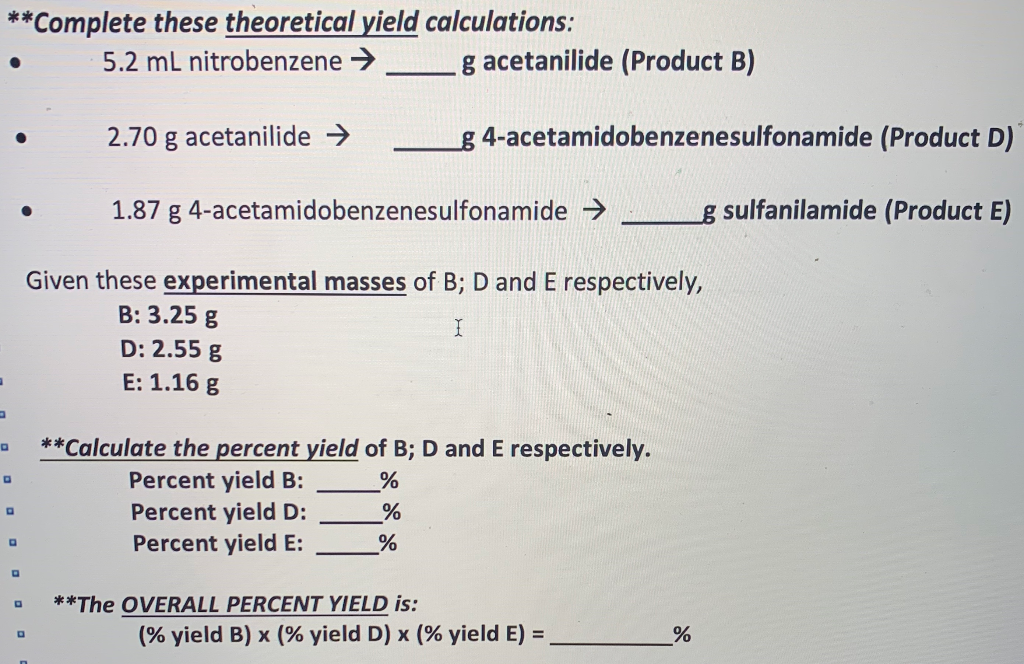
Solved Complete These Theoretical Yield Calculations 5 2 Chegg Com
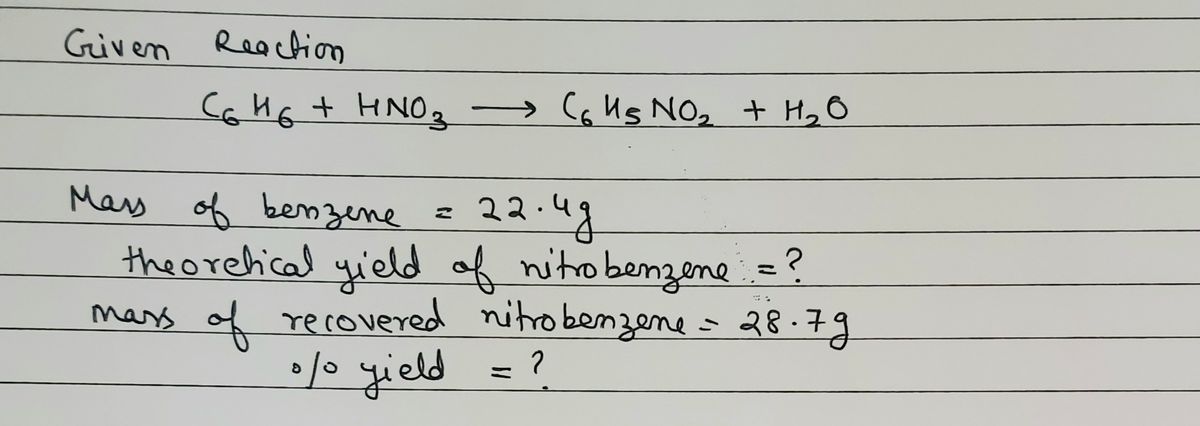
Answered Nitrobenzene C6h5no2 Is An Important Bartleby

How To Calculate Percent Yield In Chemistry Teaching Chemistry Physical Chemistry Chemistry
Answered Nitrobenzene C6h5no2 Is An Important Bartleby

Oneclass Calculate The Theoretical Yield Of C6h5no2 For This Reaction Once You Convert 28 0 G Hnos A

How To Calculate Theoretical Yield 12 Steps With Pictures
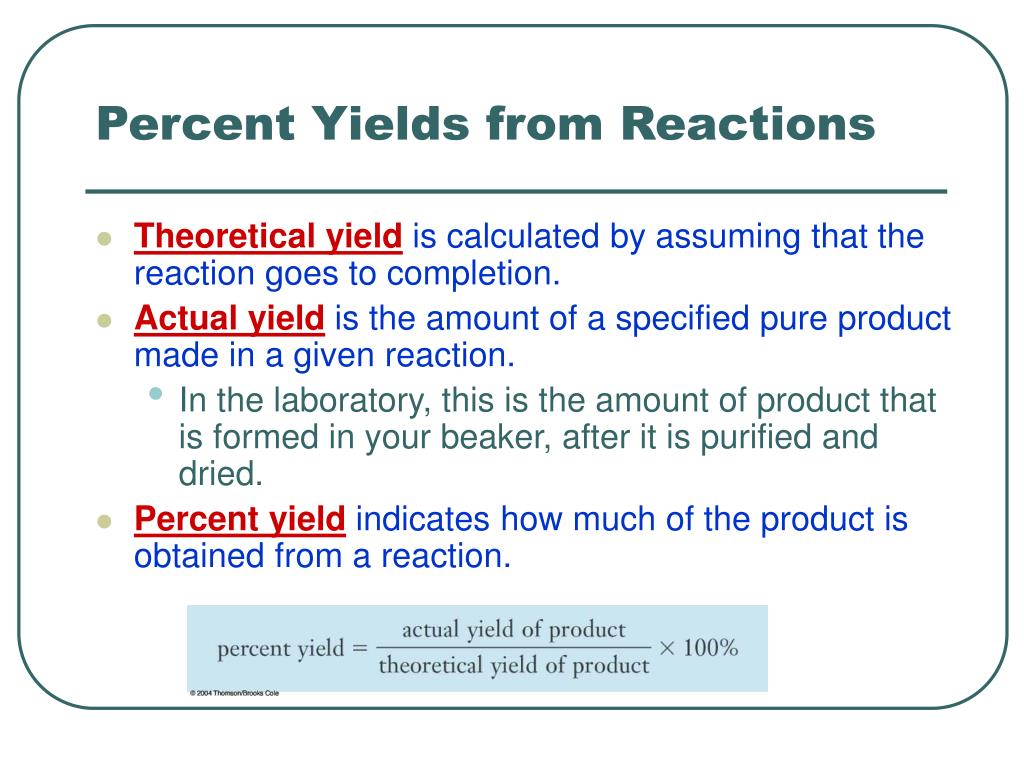
Ppt Percent Yields From Reactions Powerpoint Presentation Free Download Id 4980141

How To Calculate Theoretical Yields Youtube
Solved Once You Convert 28 0 G Hnos And 74 G Cofle Into The Chegg Com

How To Calculate Theoretical Yield 12 Steps With Pictures

Solved Nitrobenzene Left Mathrm C 6 Mathrm H 5 Mathrm No 2 Right Is Used In Small Quantities As A Flavoring Agent Or In Perfumes But Can Be Toxic In Large Amounts It Is Produced By Reaction Of Benzene Left Mathrm C 6 Mathrm H 6
Comments
Post a Comment